Step 8: Understand your on-going responsibilities once approved
HPRB approval (expedited/full board) lasts for one year.
During that time, you are responsible for:
- Requesting approval from the HPRB for any changes to your proposal
- Reporting to the HPRB any adverse events that occur while conducting your research
- Responding in a timely fashion to the annual renewal notices sent out to every researcher with an open project
- Notifying the HPRB when you have completed the research
If at any time you wish to make any change to the research proposal—including participants, procedures, instruments, and/or investigators—originally approved by the HPRB, you must submit an Amendment request within Mentor. You may not initiate any of these changes until the request has been reviewed and approved by the HPRB.
Proposals approved under expedited and full board review must be renewed annually for the duration of the research. Mentor will automatically generate a reminder prior to the expiration of your approval. You must complete an online Continuing Review application to renew your approval (or submit a Completion Report; see below). Do this within your proposal in Mentor.
While conducting your study, you may cause or experience events whose nature, severity and/or frequency are not described in the proposal approved by the HPRB.
Examples include, but are not limited to:
- unexpected complications in a participant
- missteps in the study procedures or consent documentation
- breaches of confidentiality
Alternatively, you may encounter problems or events that are potentially harmful to either the participants or the researcher.
In either case, these facts must be reported immediately to the HPRB through Mentor using an Adverse Events report.
As soon as data collection and analysis have been completed, you must report this in the Mentor system using a Completion report.
To submit a completion report in Mentor:
- Sign into Mentor and go to the HPRB tab.
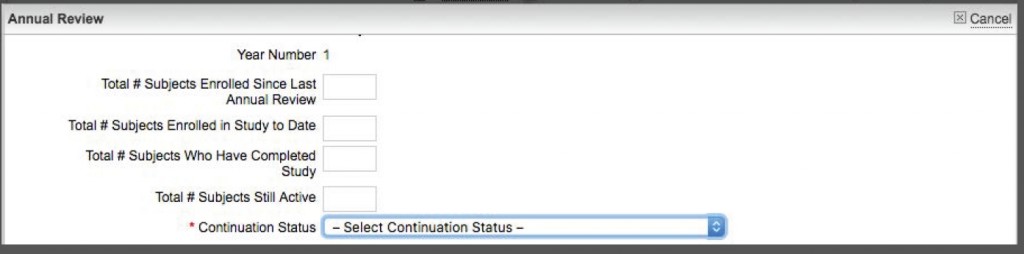
- Go to the main protocol page for your project.
- At the bottom, you should see a tab for Annual Review. Just under that is a gray button that says “Complete & Submit.” Click on that and answer the questions in the short form (see below).
- At the bottom of the Completion form, there is a drop-down menu on the right side for “Continuation Status.” From the menu, select “Terminate Protocol.”
- When you have selected “Terminate Protocol,” that then displays a “Date Proposal Closed” field where you should set the date the protocol is formally closed (terminated in our language). Save the form.
You must store signed consent documents and other study materials/data in a way that ensures confidentiality of participants’ information. Consent documents should be kept for 3 years and other materials should be kept for as long as you indicated in your proposal.
PLU will maintain documentation of HPRB activities. This documentation is kept on file for at least 3 years after completion of the research and includes:
- copies of all research proposals reviewed
- approved sample consent documents
- progress reports
- reports of injuries to subjects
- minutes of HPRB meetings
- copies of HPRB correspondence
- a list of HPRB members
- copies of policies and guidelines.